- Visibility 62 Views
- Downloads 4 Downloads
- DOI 10.18231/j.johs.2024.030
-
CrossMark
- Citation
Role of calcium sucrose phosphate (CaSP) in dental carries
- Author Details:
-
Abhijit Anil Trailokya *
-
Amar Shirsat
Introduction
Oral health is an essential component of overall well-being is often neglected across world and specially in India. Dental caries, commonly known as tooth decay or cavity, is among the most widespread oral diseases globally. It is one of the prime causative agents of oral discomfort and reason for patients to visit dental clinics or hospitals. According to the WHO, proximally 60–90% of children and nearly 100% of adults worldwide have had caries.[1]
In India overall prevalence of dental caries was 54.16%, whereas age-specific prevalence was 62% in patients above 18 years and 52% among 3–18 years of age. Maximum overall prevalence was noted in mixed dentition (58%). Region wise prevalence was more in western India (72%).[2]
Dental carries are multifactorial disease and one of the important cause is an imbalance between minerals loss (demineralization) and minerals gain (remineralization) from saliva. Microorganisms, substrate, host/teeth and time are the main factors leading to the terminal stage of continuous minerals loss is marked by the destruction of tooth structure, as known as “dental caries”. The acidic attack from cariogenic bacteria found in dental plaque biofilm growth over the tooth is responsible for the consecutive net minerals loss.
Demineralization
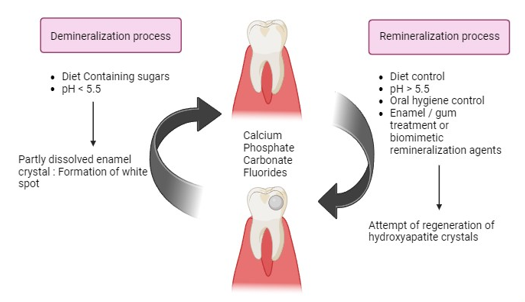
Demineralization refers to the depletion of mineral ions from the hydroxyapatite (HA) crystals that compose the hard tissues of the teeth, including enamel and dentin. If not addressed, this can result in dental cavities. Demineralization takes place when the pH level of the plaque drops below 5.5, often caused by the creation of acids by plaque microbes as they break down dietary carbohydrates. The acids dissolve the mineral content of HA crystals, resulting in the enlargement of the inter crystalline space and the increased softness and porosity of the enamel surface. White spot lesions may occur, indicating the initial stages of tooth decay, characterized by the presence of opaque white patches on the enamel surface.[3], [4]
Factors that contribute to demineralization include[3], [5], [4]

The following are preventive measures against demineralization
Fluoride usage: Fluoride has the ability to improve the process of remineralization and increase the enamel's resistance to acid erosion.
Remineralizing toothpaste: Remineralizing toothpaste contains fluoride, calcium, and phosphate, which are necessary for the process of restoring minerals to the tooth enamel. Regular utilization of these substances might facilitate the provision of essential minerals required for the restoration and fortification of tooth enamel, hence enhancing its resistance against further acid assaults.
Dietary regulation: Restricting the consumption of sweets and acids can decrease the likelihood of demineralization.
Oral hygiene: Consistently brushing and flossing helps eliminate plaque and decrease the likelihood of acid formation.
Saliva stimulation: Saliva stimulation can be achieved by chewing sugar-free gum or using other techniques. This helps to neutralize acids and supply minerals for the process of remineralization.
Remineralization
Remineralization refers to the redeposition of minerals into the crystalline structure of enamel. Demineralization occurs when the pH level in the mouth decreases as a result of consuming acidic foods or beverages or due to bacterial metabolism. This leads to the loss of minerals such as calcium and phosphate from the enamel. Failure to restore the pH level to a more neutral state might perpetuate the demineralization process, resulting in enamel deterioration and the development of caries (cavities).[6]
Remineralizing agents are substances that can counteract this process by supplying the essential minerals needed to restore the enamel prior to the formation of cavities.[6]
Broad classification of remineralizing agents, which includes

Remineralization is important because
It repairs early caries: Initial non-cavitated lesions can often be remineralized, preventing the progression to cavities.
It strengthens enamel: Remineralization creates acid-resistant crystals that are less soluble than the original enamel.
It maintains tooth integrity: By restoring minerals to enamel, remineralization helps maintain the strength and function of teeth.
Role of Calcium Sucrose Phosphate
Calcium sucrose phosphate, also known as anticay, is a versatile remineralizing agent that plays a crucial role in strengthening teeth and preventing decay. Calcium sucrose phosphate is a combination of three essential components: calcium, sucrose, and phosphate. High amounts of soluble calcium and phosphate ions are supplied by the Calcium Sucrose Phosphate. It is composed of 15% by weight of inorganic calcium orthophosphate and 85% by weight of calcium salts of phosphoric acid esters of sucrose.[7], [8]
|
Author/ Year/ Objective |
No of patients / Study duration |
Treatment received |
Parameters assessed |
Results |
|
Menon LU et al (2018) To evaluate and compare the efficacy of “calcium sucrose phosphate” (CaSP) toothpaste (5%) with ordinarily used calcium, phosphate‑containing toothpaste in elevating the level of calcium, phosphate ions in saliva. Secondary aims were to evaluate substantivity and plaque‑reducing ability of CaSP toothpaste |
N=30 participants of age group 6–13 years Values were analyzed over a period of the 12‑month study |
Group X (Control group) was made to continue brushing with their regularly used calcium, phosphate‑containing toothpaste and Group Y (Test group) was allotted CaSP toothpaste |
Alteration in the salivary calcium, phosphate level. Substantivity and plaque‑reducing ability of the two therapies |
CaSP toothpaste showed - An increase in the salivary calcium level though not statistically significant - A consistent level of calcium, phosphate in samples collected immediately and 6 h post brushing, indicating its substantivity. - Reduce the plaque level when the 1st‑month plaque score was compared with the 12th‑month score |
|
Karad A, Dhole P (2019) To evaluate the remineralizing efficacy of CaSP to determine the potential of CaSP as a remineralizing agent in increasing the microhardness of enamel and arresting the white spot lesion |
Out of 2,876 articles, only 13 were qualified for inclusion |
Effect Casp was compared with casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), casein phosphopeptide-amorphous calcium phosphate with fluoride (CPP-ACPF) and other remineralizing agents on the tooth (invitro) |
The primary outcome was to assess the remineralizing efficacy of CaSP, The secondary outcome was to assess the potential of CaSP in arresting white spot lesion(s) on enamel |
Ten studies reported an increase in the microhardness of enamel after CaSP application, and three studies reported the effect of CaSP on arresting white spot lesion(s). CaSP was found to be a better remineralizing agent in comparison to casein phosphopeptide-amorphous calcium phosphate (CPP-ACP), casein phosphopeptide-amorphous calcium phosphate with fluoride (CPP-ACPF) and other emineralizing agents in terms of increasing microhardness of enamel. In addition, CaSP application was also found to have a beneficial effect in restoring the color of white spot lesion(s) to that of normal enamel. |
|
Sargod SS et al (2015) To evaluate the remineralization potential of calcium sucrose phosphate on artificially demineralized human enamel using polarized microscopic study (in vitro study) |
20 sound human mandibular premolars subjected to demineralization in a demineralizing solution for 10 days followed by remineralization with application of ENAFIX toothpaste for 2 minutes twice daily and placed in artificial saliva for 10 days |
Effect of Casp on the tooth (invitro) |
The mean depth of penetration of lesion before and after remineralization was calculated |
The mean depth of penetration of artificial carious lesion (demineralization) was 147.5 μm and after remineralization it was seen to be 141.8 μm, the study showed significant difference (p<0.001) |
|
Myers D, Lyon TC Jr (1986) A method of improving the appearance of fluorosed teeth using Calcium sucrose phosphate gel was evaluated |
Seventeen patients with fluorosis or fluorosis-like enamel surfaces on 44 teeth were treated 2 to 4 weeks after treatment patients were evaluated |
Treatment involved cleaning the affected teeth with pumice and glycerin, rinsing with water, and applying 37% phosphoric acid for 1½-2 min. This treatment was repeated followed by application of 2% sodium fluoride for 4 min. Finally, a thick layer of 40% calcium sucrose phosphate gel was placed on the treated teeth. The patient was instructed not to rinse or eat for 30 min |
Photographs of the teeth Were evaluated pre- and posttreatment |
All 3 examiners consistently rated 10 patients as having an improved appearance. Nine of these patients were between the ages of 7 and 15 years Examiners consistently found no improvement on 2 individuals. Most of the treated teeth showed an improvement in appearance The teeth of younger children seemed to have responded better to treatment than those of adults. Only 1 of 3 adults treated showed improvement. Three independent examiners selected the posttreatment slide as an improved enamel surfacecondition in an average of 82% of the cases |
|
Raghu TN, Ananthakrishna S (2016) To evaluate the effect of calcium sucrose phosphate (CaSP) application on demineralized enamel using energy dispersive X-ray spectroscopy (EDX) and scanning electron microscope (SEM). |
20 non-carious pre-molars in patient between 18 and 25 years of age were studied before and after application of CaSP |
Effect of Casp on the tooth (invitro) All samples were brushed with Toothmin CaSP cream twice a day till 21 days and all examinations were repeated at 7, 14, and 21 days |
Artificially produced carious lesions and microhardness was rechecked to determine the remineralization post treatment |
Baseline microhardness (357.9 ± 31.2) reduced to 130.5 ± 60.4 after demineralization procedure (P < 0.001) EDX ratio increased from 2.32 ± 0.09 at baseline to 2.36 ± 0.09, 2.34 ± 0.23, and 2.44 ± 0.20 after 7, 14, and 21 days, respectively (P < 0.001). A significant increase in Ca/P ratio was found after remineralization (P < 0.001). The SEM analysis showed significant surface smoothness in 3/5 samples after 7 days and 5/5 samples after 14 days. Further improvement in surface changes of two samples and no change in other three was noted after 21 days. |
|
Dixit A et al (2021) To evaluate the remineralizing potential of three different remineralizing pastes on enamel that has been demineralized |
Sixty healthy mandibular single-rooted human premolars extracted for orthodontic reasons from subjects between 18 and 25 years of age were included |
60 premolar teeth were randomly allocated to the following three groups (20 in each group) depending on the remineralizing paste used for application as group I: bioactive glass constituting remineralizing paste; group II: tricalcium phosphate (TCP) comprising remineralizing paste; and group III: calcium sucrose phosphate (CaSP) remineralizing paste |
Artificially produced demineralization was rechecked to determine the remineralization post treatment with confocal laser scanning microscope |
The mean areas of demineralization were slightly more (133.24 ± 0.09) in the remineralization paste comprising bioactive glass seconded by the remineralization paste having CaSP (131.39 ± 0.18), and lastly the remineralizing paste constituting TCP (129.59 ± 0.14). Maximum areas of remineralization were found in the pastes that had CaSP group (96.14 ± 0.04), next by the paste having bioactive glass group (102.18 ± 0.17), and then the remineralization paste constituting TCP (118.37 ± 0.21). The difference was statistically significant among the three remineralization pastes used. |
Dental caries, often known as cavities, occur when the enamel, dentin, or cementum of the teeth are demineralized as a result of acid generation by bacterial populations. The production of these acids is a result of the metabolic breakdown of carbohydrates from the food. This process leads to the dissolution of the mineral component of the tooth, causing the release of calcium and phosphate ions. Remineralization refers to the redeposition of calcium and phosphate ions inside the decayed area. This procedure results in the formation of hydroxyapatite crystals that are bigger and more resilient against the corrosive effects of demineralization caused by acid. Calcium Sucrose Phosphate participation is vital in this process as it supplies calcium and phosphate ions that are utilized for the remineralization process.[7], [8] ([Figure 3])
Calcium sucrose phosphate is regarded as a superior agent for remineralization when compared to others due to various reasons:
High Concentration of Ions: Calcium sucrose phosphate generates an aqueous solution with a substantial concentration of calcium (10-12%) and phosphate (8-10%) ions by weight, without causing precipitation. This is crucial for the process of remineralization.
Solubility: Calcium sucrose phosphate is highly soluble in water, resulting in a significant release of calcium and phosphate ions, surpassing the typical levels found in saliva. The great solubility of these ions guarantees their easy accessibility for the process of enamel remineralization.
Plaque Inhibition: Calcium sucrose phosphate effectively hinders the development of dental plaque, a favorable outcome as plaque can serve as a breeding ground for acid-producing bacteria, resulting in demineralization.
Enhanced Enamel Properties: Research has demonstrated that Calcium sucrose phosphate can augment the microhardness of enamel, indicating that it not only facilitates remineralization but also improves the quality of the enamel surface.
Long-Term Effectiveness: Calcium sucrose phosphate has demonstrated substantial remineralization effects throughout a timeframe of 7 to 14 days after treatment, suggesting its ability to consistently promote remineralization over an extended period [9], [10].
Evidence with Calcium sucrose phosphate.[11], [12], [13], [14], [15], [16] Refer [Table 1].
Discussion
Dental caries, despite various modern advancements, remains as one of the most widespread pandemic diseases worldwide. A combination of diet, pathogenic bacteria (plaque), and unfavorable salivary components together leads to production of organic acids which results in drop in pH, eventually leading to demineralization and cavitation. Fluoride has been considered the cornerstone of remineralization. Studies have shown that fluoride drives the process of remineralization only if there is adequate supply of calcium and phosphate. Further, along with calcium and phosphate, calcium sucrose phosphate (CaSP) addition was studied which also showed synergistic benefits. Anticay, a CaSP–calcium orthophosphate complex, supplies both calcium and phosphate in a soluble form. This complex helps reduce the acid solubility of enamel and increase the rate of remineralization by a common ion effect. Furthermore, it has shown to inhibit the formation and adherence of plaque to the enamel surface.
Conclusion
Calcium sucrose phosphate (CaSP) is an effective agent in the prevention and management of dental caries. Its primary function is to facilitate the remineralization of tooth enamel by providing bioavailable calcium and phosphate ions. CaSP may help in maintaining a neutral pH in the oral environment, inhibiting plaque formation, and enhancing enamel resistance to acid attacks thus Calcium Sucrose Phosphate in toothpaste strengthened the enamel. CaSP toothpaste also showed substantivity and plaque-reducing ability. When used in combination with fluoride, CaSP’s efficacy in preventing dental caries is further enhanced. Due to its non-toxic nature and additional benefits in oral health, CaSP is a valuable component in dental care products aimed at reducing the incidence of dental caries.
Source of Funding
None.
Conflict of Interest
Dr Abhijit Anil Trailokya & Dr. Amar Shirsat are associated with Indoco Remedies Ltd.
References
- PE Petersen, D Bourgeois, H Ogawa. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005. [Google Scholar]
- P Pandey, T Nandkeoliar, A Tikku, D Singh, MK Singh. Prevalence of Dental Caries in the Indian Population: A Systematic Review and Meta-analysis. J Int Soc Prev Commun Dent 2021. [Google Scholar]
- A Anil, WI Ibraheem, AA Meshni, R Preethanath, S Anil, A Anil. Demineralization and Remineralization Dynamics and Dental Caries. Int J Nanomed 2016. [Google Scholar]
- EAA Neel, A Aljabo, A Strange, S Ibrahim, M Coathup, AM Young. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomed 2016. [Google Scholar]
- PN Geethu, N Suvedha, AA Kurien, Y Chakravarthy, V Pallavi. Demineralization and remineralization in restorative dentistry. Acad Dent Educ 2022. [Google Scholar]
- MK Arifa, R Ephraim, T Rajamani. Recent Advances in Dental Hard Tissue Remineralization: A Review of Literature. Int J Clin Pediat Dent 2019. [Google Scholar]
- LU Menon, RB Varma, P Kumaran, AM Xavier, BS Govinda, JS Kumar. Efficacy of a Calcium Sucrose Phosphate Based Toothpaste in Elevating the Level of Calcium, Phosphate Ions in Saliva and Reducing Plaque: A Clinical Trial. Contem[ Clin Dent 2018. [Google Scholar]
- TM Titty, SB Shrikrishna, A Rao, R Shenoy, S Natarajan. Remineralizing Effectiveness of Calcium Sucrose Phosphate and Fluoride Dentifrices: An In vitro Study. Contem Clin Dent 2018. [Google Scholar]
- D Vinod, A Gopalakrishnan, SM Subramani, M Balachandran, V Manoharan, A Joy. A Comparative Evaluation of Remineralizing Potential of Three Commercially Available Remineralizing Agents: An In Vitro Study. Int J Clin Pediat Dent 2020. [Google Scholar]
- P Aishwarya, DS Dinakaran. Evaluation of caries remineralization potential of toothpastes containing three remineralizing agents - calcium sucrose phosphate, β tricalcium phosphate and fluoro calcium phosphosilicate: An in vitro study. Int J Appl Dent Sci 2023. [Google Scholar]
- LU Menon, RB Varma, P Kumaran, AM Xavier, BS Govinda, JS Kumar. Efficacy of a calcium sucrose phosphate based toothpaste in elevating the level of calcium, phosphate ions in saliva and reducing plaque: A clinical trial. Contemp Clin Dent 2018. [Google Scholar]
- A Karad, P Dhole. Evaluation of Remineralizing Efficacy of Calcium Sucrose Phosphate: A Systematic Review of In Vitro Studies (2019). J Indian Orthod Soc 2019. [Google Scholar]
- SS Sargod, SS Bhat, S Hedge, R Karunakaran. Remineralization potential using calcium sucrose phosphate (EnaFix) on artificial carious lesion: A polaroid microscopic study.. Int J Adv Res 2015. [Google Scholar]
- D Myers, TC Lyon. Treatment of fluorosis or fluorosis-like lesions with calcium sucrose phosphate gel. . Pediatr Dent 1986. [Google Scholar]
- TN Raghu, S Ananthakrishna. Remineralization Potential of Calcium Sucrose Phosphate on Demineralized Enamel: Results of an In Vitro Study. J Int Oral Health 2016. [Google Scholar]
- A Dixit, MO Mampilly, R Nallamothu, R Gopakumar, M Jayachandran, NM Terence. Analysis of Remineralization Potential of Three Different Remineralizing Pastes on Demineralized Enamel: A Comparative Study. J Contemp Dent Pract 2021. [Google Scholar]
- Introduction
- Demineralization
- Factors that contribute to demineralization include[3], [5], [4]
- The following are preventive measures against demineralization
- Remineralization
- Broad classification of remineralizing agents, which includes
- Remineralization is important because
- Role of Calcium Sucrose Phosphate
- Discussion
- Conclusion
- Source of Funding
- Conflict of Interest
