- Visibility 42 Views
- Downloads 3 Downloads
- DOI 10.18231/j.johs.2024.021
-
CrossMark
- Citation
Scaffolds in periodontal regeneration- A brief review
Introduction
The periodontium is the supporting structure of tooth formed by the integration of periodontal ligament, cementum, gingiva and alveolar bone. The health of periodontium gets affected after the ingress of bacteria by local or systemic causes. Most common cause of periodontal diseases is plaque accumulation around the teeth. Dental plaque serves as a substrate for several bacterial species. If dental plaque is not removed it tend to initiate inflammatory reaction that may hamper the health of periodontium. The disease further progresses to the level of junctional epithelium resulting in bacterial invasion into the attachment apparatus ultimately leading to the attachment loss. Conventional therapy, surgical and non-surgical, for treating periodontal diseases focuses only on the removal of causative agent i.e bacteria. This concern led to the introduction of various biomaterials to promote the tissue regeneration, guided tissue regeneration (GTR) being one on them. Although such treatment gives promising results in terms of disease prevention but the desired attachment level cannot be attained. Therefore primary aim of periodontal therapy should be to restore the form and function of periodontium. In 1993 the concept of tissue engineering was proposed by Langer.[1] This concept bridges the interface between science and host tissue biocompatibility . Moreover, recent advances like incorporation of stem cells, bioactive molecules in tissue engineered scaffold serves to amplify the outcomes of regeneration therapy.[2]
The three key components of engineered tissues are :
Progenitor cells
Scaffold or supporting matrix
Signaling molecules.[3]
These elements are commonly referred to as tissue engineered triad. Each element has a specific function to perform. The progenitor cells promotes the synthesis of new tissues, whereas scaffolds provides the favourable environment for progenitor cells to perform their action. Additionally, signalling molecules acts as a guide to promote growth of certain cells in a definite pattern so as to achieve desired results. Bone grafts are introduced to serve the purpose of regeneration of tissues and various materials are used to date including such as autogenous bone and its substitutes. Although, bone grafting with autogenous bone is considered as the golden standard because of its unique osteoconductive, osteoinductive, and osteogenic properties , it is very difficult to harvest the bone and an additional requirement of surgical procedure allograft and xenografts become the preferred choice.[4] Additionally, the risk of rejection is minimized in case of allografts and xenografts due to their biocompatibility and composition.[5] It exhibits the property of osteoconductivity which holds an added advantage as a material as it stimulates the progenitor cells formation.[6] However, a systematic review has concluded that when the periodontal therapy is done with bone grafting alone, without membranes, cementum and periodontal ligament regeneration will remain absent.[7]
Background of Periodontal Regeneration Therapy
Healthy periodontuim consists of junctional epithelium(JE), cementum(CM), connective tissue(CT) and alveolar bone (AB)as depicted in [Figure 1] A. Periodontitis is an inflammatory disease initiated by bacterial invasion and eventually progresses as a result of the cascade of inflammatory response generated involving neutrophils infiltration followed by tissue destruction and bone resorption carried out by macrophages and osteoclasts activated in response to inflammation ([Figure 1] B).[8]
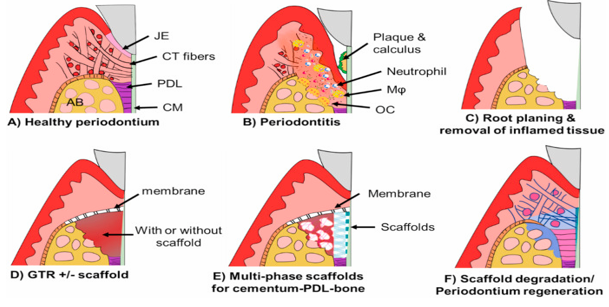
[Figure 1] C described the conservative approach, including scaling, root planning and curettage, where main aim of the therapy is to remove the plaque and calculus. No attachment gain is attained with this approach as healing takes place in the form of long junctional epithelium and not by new attachment. Therefore, chances of recurrance of periodontitis is likely to happen especially if patients fails to maintain the oral hygiene or in patients with immune-compromised diseases. This concern led to the introduction of bone grafting procedures in periodontics. Although, bone graft procedures were performed in order to prevent the healing by long junctional epithelium but bone graft alone does not show promising results. Therefore, the concept of GTR was introduced ([Figure 1] D) with the principle of excluding the unwanted cell lines, epithelial cells , from the site of healing thus promoting the growth of necessary cells to achieve desired results.[9] The concept of GTR was started by Karring and Nyman in the 1980s. In 1982, Nyman et al. examined the regeneration potential of PDL cells in monkey and reported that healing by new attachment can be achieved when periodontitis –affected tooth is treated with GTR and reported ankylosis and root resorption in some cases. Inspite of promising results, few shortcomings were reported, which brings about proposal of concept of tissue engineering in the field of periodontics so as to expedite tissue regeneration tissue engineered scaffolds are fabricated with the incorporation of stem cells or bioactive materials along with GTR ([Figure 1]D). Recently, tissue engineered-scaffolds are generated to guide integrated regeneration of periodontium ([Figure 1] E). These scaffolds are designed such that they undergo the process of degradation and provide a medium for the regeneration of periodontal tissues ([Figure 1] F).
Scaffolds
Scaffolds are the three-dimensional structures or matrices that provides the environment to induce the tissue formation.[10]
Scaffolds contribute to the formation of new tissues by following actions:
Provides a 3-dimensional framework in order to provide physical support thus maintaining the shape of defect.
Promote the migration and attachment desired cell-type and also inhibits the other cell lineages to migrate in the area of regeneration in the 3-dimensional matrices.
Deliver the required nutrients as well as growth factors to the site of action.
Cell source for progenitor Cells
Over the last decade, the use of progenitor cells in tissue-engineering has markedly increased due to its regenerative capacity. Various approaches are proposed for cell‑based periodontal regeneration and gave promising results.[3], [4], [5]
Periodontal ligament‑derived cells
Periodontal tissue stem cells found periodontal ligament cells are also referred to as mesenchymal/ stromal cells. These cells have therapeutic potential as they are pleuripotent, highly proliferative and easy to obtain. The periodontal ligament‑derived mesenchymal stromal cells are known to promote the formation of new periodontal tissues. Nakahara et al. [11] studied the regenerative potential of periodontal ligament-derived cells periodontal tissues by placing periodontal ligament cell scaffold in dogs and reported the neoformation of periodontal tissues .Seo et al.[12] stated that periodontal ligament‑derived cells exhibits pleuripotency effect, high turnover rate and clonegenicity.
Periosteal cells
The periosteal cells possess the ability to differentiate into fibroblasts, osteoblasts, chondrocytes, adipocytes and skeletal myocytes. Moreover, these may induce the formation cementum, alveolar bone and periodontal ligament. Owing to this multipotent property of periosteal cells, they are being used in tissue engineering.[13]
Gingival epithelium and fibroblast
The approach of tissue engineering is to stimulate the growth of tissue in a uniform direction in order to achieve simulation of original periodontal tissues. Gingival epithelium and gingival fibroblasts are used to serve this purpose. These cells enhances the process of repair due to timely release of growth factors. As a result, gain in epihtelial attachment is observed in such cases. Mohammadi et al.[14] evaluated the width of keratinised gingiva in cases where gingival epithelium graft and recorded increase in width of keratized gingiva.
Bone marrow‑derived mesenchymal stem cells
Bone marrow derived mesenchymal stromal cells are known to exhibits the property of proliferation and differentiation into multiple lineages of cells. They also have a propery of tissue guided differentiation that potentiates its use as a cell source for tissue engineering. in a study performed by Kawaguchi et al.[15] showed the promising results and enhanced regeneration in furcation defects.
Scaffold biomaterials for periodontal regeneration
Ceramics
Ceramics are used in tissue engeneering due to its biocompatible nature and osteoconductive property. More oftenly used ceramics are hydroxyapatite (HA), and beta tricalcium phosphate (TCP). They are not known to stimulate immunological reaction because of their protein free composition. HA (hydroxyapatite) was among the first used scaffold biomaterial. It can be derived from variety of synthetic materials that may be from bovine bone or coralline.[16] TCP is composed of calcium and phosphorus and consequently can be used as a substitute to the bone .it is one of the naturally obtained biomaterial.
Polymers
These include synthetic as well as natural polymers. Most oftenly considered natural polymers are collagen fibrin, albumin, hyaluronic acid, cellulose, chitosan, polyhydroxyalkanoates, alginate, agarose and polyamino acids.[17] Examples of commonly used synthetic polymers are polyglycolic acid, polylactic acid and polycaprolactone. Natural polymers include.
Synthetic polyesters
Synthetic polyesters have been pivotal in tissue engineering, with PGA (polyglycolic acid) emerging as the pioneering polymeric scaffold. Despite its insolubility in water, PGA is prone to hydrolysis. Conversely, PLA (polylactic acid), derived from lactic acid, exhibits greater hydrophobicity and resistance to hydrolysis than PGA. A significant advancement is PLGA (polylactic; glycolic acid), a copolymer of PGA and PLA. Renowned for its biocompatibility, controlled structural and mechanical properties, tailored degradation rates, and potential as growth factor delivery vehicles, PLGA stands out as a leading candidate for applications in regenerative medicine and dentistry.[18]
Natural polymers
Nowadays natural biomaterials are considered as scaffolds for tissue regeration; They are highly biocompatible, less toxic and very rarely cause immune reactions. The two most frequently used natural biomaterials are collagen and chitosan for periodontal tissue regeration.[16]
Chitosan
Chitosan has also been widely preferred as a material for periodontal tissue regeneration. Owing to its non ;toxic, antimicrobial, non‑immunogenic and excellent biocompatible nature it is feasible to use it as substitute to the bone.[17]
A biodegradable natural carbohydrate biopolymer has demonstrated associations with enhanced healing and bone formation. Its structural characteristics render it practical for utilization as a scaffold for new attachment. [18]
Collagen – the collagen scaffold is a 3 D network of collagen which is porous through which calls can migrate and perform regenerative functions various forms of collagen are used as scaffold:
Collagen foam – The fabrication is done by freeze drying a solution of collagen which is then placed in a mold to get desired configuration. In order to make to scaffold more stable resistant to contraction physical or chemical cross‑linking of sufficient duration and intensity is achieved. Based on this cross-linking regimen, foam scaffold possess decreased or increased resistance to breakdown by collagenase. [19]
Collagen fiber –Owing to its complex arrangement single oriented collagen fiber scaffolds are not used widely in tissue engineering. To overcome this shortcoming collagen fiber scaffold with 3 D complex structure was successfully developed. Commercially Fibers of 300‑nm diameter and above have been fabricated. To make it more resistant to collagenase as compared to gel ore foam form cross-linking should be done such that ideal 67 nm cross banding should not be violated . [20]
Collagen membrane scaffolds - Collagen membranes are fabricated by drying collagen solutions on surfaces such as Teflon or polyethylene. The process involves neutralizing and warming the solution to 37ºC, which facilitates collagen polymerization and fibril formation. To prevent premature gelling, the solution is dried on a suitable surface. Various methods can enhance the wet strength of these membranes through crosslinking. For instance, aldehydic crosslinking inhibits cell attachment, while UV crosslinking decreases resistance to collagenase.[21]
Hydrogel
Hydrogel constitutes a network of crosslinked macromolecular polymers with hydrophilic properties and absorption characteristics. Various biomaterials can be fashioned into hydrogel forms.[22] The advantages of hydrogel include its high-water content, biocompatibility, and flexibility in structural design and formation.
In treating class II furcation defects, collagen hydrogel scaffolds loaded with FGF-2 CM-like tissue and PDL-like Sharpey's fibers facilitate tissue formation without ankyloses and root resorption. The ease of preparation for growth factor-loaded or cell-encapsulated hydrogels makes them preferable as carriers for growth factors, cytokines, and/or cells. However, it's important to note that the release of growth factors loaded in hydrogels can vary depending on the chemistry, degree of crosslinking, and degradation kinetics of the hydrogel(s), as well as interactions between the hydrogel and drug or growth factor molecules.[23] The release rate may significantly differ between in vitro and in vivo settings. Therefore, it's crucial to consider a targeted controlled release pattern of bioactive cues delivered through hydrogel-based scaffolds.
A primary limitation of hydrogel in tissue engineering applications is its weak mechanical stability. To overcome this limitation, several studies have explored chemical modifications or the creation of hybrid scaffolds with structural polymers.
3D-printed scaffolds
3D printing represents an emerging technology facilitating precise control over the macro- and micro-structure of tissue engineering scaffolds.[24] The periodontium, a complex structure comprising various tissue types including soft and hard tissues, requires a multi-phase tissue composition for effective recapitulation. Recently, the adoption of 3D printing techniques has allowed for the fabrication of scaffolds with regionally variant internal microstructures suitable for cementum, periodontal ligament (PDL), and/or alveolar bone (AB).[25] Moreover, different growth factors can be incorporated into 3D printed scaffolds to aid in the regeneration of each tissue within the periodontium.
Beyond internal microstructure, 3D printing, through layer-by-layer deposition, facilitates the creation of custom-designed scaffolds tailored to the specific shape and dimensions of individual periodontal defects.[26] Despite the relatively small number of publications to date, the application of 3D printing for periodontal regeneration shows promising growth.
Signalling molecules
Signaling molecules play a crucial role in enhancing the in vivo efficacy of scaffolding materials for periodontal wound repair in both preclinical and clinical studies.
Platelet Derived Growth Factor (PDGF): It is a dimeric molecule composed of two peptide chains, termed A and B chains, and is recognized as a potent mediator of periodontal tissue regeneration. PDGF exhibits chemotactic and mitogenic properties for periodontal ligament cells and stimulates gingival fibroblast hyaluronate synthesis, providing a lattice for extracellular matrix formation. Furthermore, PDGF enhances bone and cementum formation by downregulating alkaline phosphatase activity. Moon II Cho (1995) [27]conducted an animal study on beagle dogs and introduced PDGF modulated guided tissue regeneration therapy, concluding that PDGF BB modulated therapy promotes periodontal regeneration more rapidly and effectively compared to GTR alone. Recombinant human platelet-derived growth factor BB homodimer is approved for the treatment of periodontal defects and is commercially available as Gem 21 (Osteohealth, Shirley, NY).
Fibroblast Growth Factor (FGF): It belongs to the heparin-binding growth factor family and possesses angiogenic properties. There are seven forms of fibroblast growth factor, including Acidic FGF (a FGF) and basic FGF (b FGF). It is found in various cell types such as smooth muscles, endothelial cells, chondrocytes, and osteoblasts. Owing to its mitogenic property it is known to have profound effect on healing of bone and periodontal tissues for fibroblasts. Terranova (1989)[28] et al. demonstrated that enhanced wound healing and regeneration of periodontal ligament tissues when treated β FGF. Till now,15 different types of proteins are identified as a part of transforming growth factor β superfamily.
Bone Morphogenetic Proteins
Bone morphogenetic protein 2 (BMP-2) exists as a disulfide-linked homodimer. BMP-2 plays a pivotal role in promoting periodontal healing by inducing the differentiation of undifferentiated pluripotent cells into desired cell lineages, including cartilage and bone-forming cells. Alongside β-FGF, it stimulates angiogenesis and enhances alkaline phosphatase activity, thus facilitating bone formation. Thorarinn J. Sigurdsson (1995) [29] conducted a study on beagle dogs with artificially created 5 mm deep bone defects, concluding that rhBMP-2 treated sites exhibited higher alveolar bone levels compared to control sites.
Insulin Like Growth Factor
IGF I, also referred to as somatomedin C, and IGF II, known as multiplication stimulating activity, are prominent members of this growth factor class. IGF I is notably present in platelets at substantial levels and is released during clotting alongside other growth factors. It acts as a potent chemotactic agent for vascular endothelial cells, thereby increasing neovascularization.[30] Furthermore, IGF I promotes osteogenesis and cementogenesis, exerting a strong influence on periodontal ligament fibroblast mitogenesis and protein synthesis in vitro.
On the other hand, IGF II is the most abundant growth factor in bone tissue, also contributing to parameters of bone formation, albeit with less potency compared to IGF I. Matsuda et al. (1992) [31] demonstrated the mitogenic effects of IGF I on periodontal ligament fibroblastic cells and concluded that a synergistic effect arises from the combination of PDGF AB and IGF 1.
Transforming Growth Factor-β
It is predominantly found in bone and platelets. TGF β was initially recognized for its ability to induce non-transformed cells in soft agar. Highly specific in its action, TGF β exhibits a strong affinity for inducing the production of extracellular matrix. It selectively stimulates periodontal ligament fibroblast proliferative activity, leading to the production of type I collagen, fibronectin, and osteocalcin, ultimately resulting in bone matrix deposition.
Moreover, TGF β decreases the synthesis of metalloproteinases and plasminogen activator while increasing the synthesis of tissue inhibitor of metalloproteinases and plasminogen activator inhibitor (PAI), thereby reducing connective tissue destruction. TGF β also inhibits the formation of osteoclast-like cells. Given its pleiotropic effects on bone matrix formation and resorption, as well as its relative abundance in bone, TGF β may act as a bone coupling factor, linking bone resorption to bone formation.[32]
Periodontal Ligament Derived Growth Factor
Nishimura et al. (1995) [33] discovered a growth factor derived from the periodontal ligament known as PDL CTX, which is a polypeptide factor sourced from human periodontal cells. This peptide serves as a highly specific autocrine chemotactic agent for human periodontal ligament cells, exhibiting potency levels approximately 1000 times higher than many recognized growth factors such as IGF, PDGF, and TGF. Additionally, PDL CTX is being considered for inclusion in biological therapeutic approaches targeting cell-specific periodontal regeneration, given its lack of chemotactic effect on gingival fibroblasts or epithelial cells.
Scaffold Architectures and Fabrication Techniques
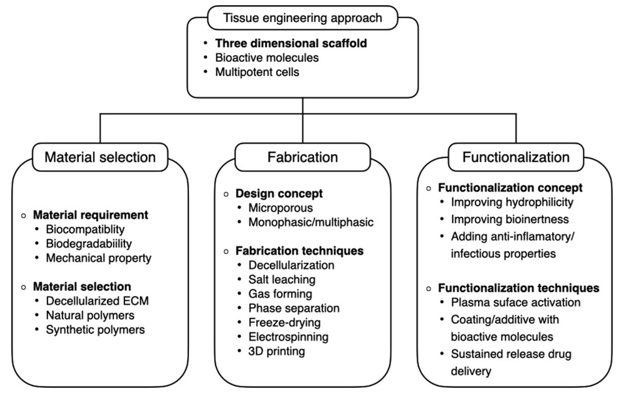
As tissue engineering in periodontics has burgeoned since its therapeutic potential was initially suggested in the mid-1990s, the number of studies in this field has grown exponentially. Advancements in material development, fabrication techniques, and digital solutions have significantly propelled this novel approach to periodontal regeneration. Numerous studies have been conducted to design scaffolds capable of guiding site-specific regeneration, with the concept centered around the production of biomimetic periodontal scaffolds ex vivo. This involves combining various materials and employing functionalization methods to create scaffolds with specific architectural patterns that provide spatial guidance to both endogenous and exogenous progenitor cells. The functionality of these cells may be further enhanced by incorporating bioactive molecules into the scaffolds.
Presently, high-resolution 3D printing technology allows for the rapid production of polymeric scaffolds in predetermined forms.[34] With the widespread use of CT scans and 3D intraoral scanners, this technique is highly compatible with dental clinical settings, enabling the production of patient-specific scaffolds either chair-side or in laboratories. Although clinical evidence regarding the efficacy of 3D-printed scaffolds is currently limited, ongoing optimization of microstructure, material selection, and functionalization to incorporate bioactive features may enhance future clinical outcomes. Moreover, advancements in bioprinting technology hold the potential for the production of patient-specific biomimetic periodontal implants.
Scaffolds play a crucial role in providing structural support and guidance for both exogenous and endogenous cells. [35] Generally, 3D scaffolds with high porosity and interconnectivity are deemed more suitable for achieving structural and functional restoration. This architectural design offers a conducive microenvironment for cell-to-cell interaction and scaffold-to-tissue integration at the implantation site.[36] During the initial phases of implantation, stabilizing the blood clot is considered a key initiator of tissue repair and regeneration, facilitated by porous structures that enable blood infiltration into the scaffolds through enhanced vascularization.
Macropores ranging from 100 to 700 micrometers promote vascularization at the implanted sites, while micropores smaller than 100 micrometers may impede cell growth due to local ischemia. Moreover, high porosity facilitates the diffusion of nutrients and gases and aids in waste removal, thereby enhancing cellular metabolism and growth. Various fabrication techniques have been employed to design highly porous scaffolds.[37]
Approaches for integrated periodontal regeneration
The recent focus of periodontal regeneration has shifted towards the effective and simultaneous regeneration of three tissues: periodontal ligament (PDL), alveolar bone (AB), and cementum (CM) in periodontal defects, independent from PDL cells used in tissue engineering and scaffold technologies. This integrated periodontal regeneration relies on four key principles: scaffolds, blood supply, cells, and signaling molecules, coupled with periodontal therapy involving the removal of biofilm and reduction of inflammation.[38]
There are several advantages to employing multi-phasic scaffolds for periodontal tissue regeneration over traditional single-phasic scaffolds. Precisely engineered spatial and temporal release of multiple signaling molecules enables direct and prolonged initiation of the regeneration pathway, thereby promoting target tissue regeneration. Periodontal tissue regeneration necessitates three-dimensional spaces for new CM, PDL, and AB. Multi-phasic scaffolds can furnish the spatial niches where new cells and tissues can effectively reside and communicate. Furthermore, the timely release of signaling molecules from multi-phasic scaffolds could offer a more controlled approach to guide regeneration of each component of the periodontium.
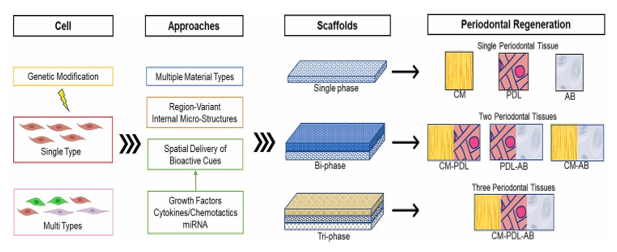
Multi-phasic scaffolds can be divided into two main categories: bi-phasic and tri-phasic designs ([Figure 3]). Each layer or phase is specifically engineered to guide the regeneration of a particular target tissue. In the context of integrated periodontium regeneration, which involves the regeneration of PDL, AB, and CM, bi-phasic or tri-phasic scaffolds are considered ideal for achieving true periodontium regeneration.[39]
Bi-phasic scaffolds comprise two distinct phases, each targeting different tissues simultaneously: PDL-AB, AB-CM, or PDL-CM. On the other hand, tri-phasic scaffolds consist of three phases that simultaneously target three different tissues: PDL-AM-CM. The utilization of multi-phasic scaffolds in periodontal tissue regeneration offers advantages over single-phasic scaffolds or traditional guided tissue regeneration (GTR) materials. This is because periodontal tissue regeneration requires three-dimensional spaces for new CM, PDL, and AB, and multi-phasic scaffolds can provide the spatial niches where new cells and tissues can effectively communicate.
Bi-layered or bi-phasic scaffolds have demonstrated effectiveness in integrated periodontal tissue regeneration by promoting the formation of CM, PDL, and AB. Similarly, tri-phasic scaffolds offer a complete set for the integrated periodontium, facilitating the regeneration of CM, PDL, and AB.
Recent advances
Custom-designed 3-dimensional scaffold for a personalized periodontal approach
A personalized medicine approach underscores the acknowledgment of pathologic variations among patients. Effective periodontal regeneration necessitates spatial guidance to progenitor cells with rich vascularization while preventing epithelial downgrowth. Consequently, 3D scaffolds have emerged as a potential enhancer of periodontal regeneration. This objective can be achieved through the utilization of medical imaging systems such as high-resolution cone beam CT scans in scaffold design.
Park and colleagues (2010, 2012)[40], [41] introduced a prototype workflow for custom-designed 3D scaffolds aimed at periodontal regeneration. In this approach, periodontal fenestration defects were surgically created and scanned using a micro-CT scan.
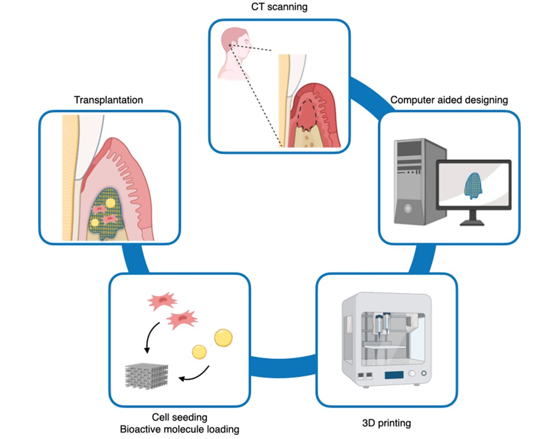
The fabrication workflow of personalized 3D-printed scaffolds for periodontal regeneration involves several steps:
CT Scanning: The geometry of the periodontal defect is obtained through CT scanning
Computer-Aided Design (CAD): The CT scan data is processed in CAD software to design a scaffold that can adapt to the specific defect.
3-D Printing: Using the CAD file, the scaffold is produced by 3D printing using the desired biomaterial. This allows for precise fabrication of the scaffold according to the specifications outlined in the CAD design.
Incorporation of Multipotent Cells and Bioactive Molecules: Multipotent cells and bioactive molecules may be incorporated into the scaffold to enhance its functionality before transplantation. These additions can help promote tissue regeneration and improve the overall effectiveness of the scaffold in facilitating periodontal regeneration.
The scanned files were then imported into CAD software as 3D image data in .stl format, where the scaffold geometry was tailored to fit the defect. Microchannel architectures were incorporated into the scaffold to provide guidance to periodontal ligament fibers. Subsequently, a wax mold was generated using a wax printer, and polycaprolactone (PCL) was cast in the mold. Following sterilization, periodontal ligament cells (PDLC) were loaded onto the custom-designed scaffold and transplanted into the defect site.
After 4 weeks of healing, the custom-designed scaffold led to a significant increase in bone mass and mineral density, with the regenerated periodontal ligament exhibiting more regular alignment compared to amorphous scaffolds with random pores produced by the freeze drying method. Periostin expression was observed at the treated site with the custom-designed scaffold but not with the amorphous scaffold. During periodontal surgery, the scaffold was soaked in recombinant human platelet-derived growth factor BB before transplantation into the defect site.
After 12 months of treatment, clinical attachment gain and radiological bone regeneration were observed without complications. However, in one instance, the entire scaffold was removed due to infection after 13 months. Histologic assessment and gel permeation chromatography revealed that a significant portion of the scaffold remained, with approximately 76% of the PCL molecular weight intact. Moreover, new bone formation was limited. This suggests that while custom-designed scaffolds may guide tissue regeneration, prognosis is contingent upon material degradation and biological interactions between materials and tissues.[42]
Further optimization of internal microstructure, polymer selection, and polymer functionalization holds promise for enhancing outcomes. Bioprinting technology has emerged as a cutting-edge tool for fabricating 3D biofunctional hierarchical architectures with one or multiple types of living cells incorporated. It imparts biological functionality to conventional 3D printed scaffolds by mimicking in vivo cell-to-cell and cell-to-matrix interactions within the structure. For future applications in periodontal regeneration, ongoing efforts are focused on optimizing bioink using periodontal ligament cells (PDLC). Currently, photo-cross-linkable hydrogels such as gelatin-methacryloyl and poly(ethylene glycol) dimethacrylate hydrogel are being explored as base materials. Optimization efforts involve assessing printability, mechanical stability, and cytocompatibility through experimentation with various extrusion parameters and crosslinking methods. While progress is in its early stages, the necessity of hierarchical regeneration suggests that bioprinting in the field of periodontal regeneration is poised to attract increasing research attention.
Discussion
Scaffolds have emerged as a pivotal component in regenerative periodontics, offering promising solutions for the restoration of periodontal tissues lost due to diseases like periodontitis. The primary goal in periodontal regeneration is to restore the complex architecture of the periodontium, which includes the cementum, periodontal ligament, and alveolar bone. Scaffolds play a crucial role in this process by providing a temporary three-dimensional structure that supports cell attachment, proliferation, and differentiation, ultimately leading to tissue regeneration.
To effectively support periodontal regeneration, scaffolds must be biocompatible, avoiding immune responses while supporting cell growth and safely degrading over time. They need to degrade at a rate that matches tissue formation and must have adequate porosity for nutrient exchange and cell attachment. Additionally, scaffolds should possess mechanical strength to withstand forces and be flexible enough to conform to periodontal structures. Lastly, they must promote bone formation (osteoconductivity) and encourage cell differentiation into osteoblasts (osteoinductivity), crucial for regenerating the alveolar bone.
One of the primary challenges in using scaffolds for periodontal regeneration is ensuring the simultaneous regeneration of all periodontal components (cementum, ligament, and bone). Achieving this requires scaffolds that can deliver multiple bioactive agents, such as growth factors and stem cells, in a controlled manner. The creation of bioactive scaffolds including growth factors such as vascular endothelial growth factor (VEGF) or bone morphogenetic proteins (BMPs) is one example of recent developments. These scaffolds improve cell signalling pathways, which actively encourage tissue regeneration. Another frontier is cell-seeded scaffolds, which are scaffolds that have progenitor or stem cells preinstalled. Because these scaffolds offer a ready population of cells that can differentiate into the required tissue types, they help hasten the regenerative process.
Scaffold design is also being revolutionised by nanotechnology and 3D printing, which enable exact control over scaffold construction and the addition of nanoscale elements that can resemble the natural ECM. These technologies make it possible to design scaffolds with extremely particular characteristics that are suited to the periodontal tissues' regenerating requirements.
Scaffolds are being tested more often in clinical settings after demonstrating encouraging outcomes in preclinical research. But overcoming from the bench to the bedside means getting over legal obstacles, making sure that findings are repeatable, and taking long-term safety concerns effectively.
Prospective avenues for scaffold-based periodontal regeneration entail the creation of adaptive scaffolds that are capable of changing their surroundings by regulating the release of bioactive substances or modifying their rate of degradation dependent on the tissue regeneration phase. Furthermore, promising is the incorporation of personalised medicine, in which scaffold attributes are adjusted to meet the demands of specific patients based on environmental and genetic characteristics.
A fundamental component of regenerative periodontics, scaffolds provide an adaptable foundation for regenerating damaged periodontal tissues. Scaffolds have significant potential for attaining successful and predictable periodontal regeneration with continuing research and advances in technology.
Conclusion and Future Perspective
Periodontal regeneration is a highly intricate process, requiring coordinated efforts to restore the complex structure of the periodontium, including the gingival epithelium, periodontal ligament, cementum, and alveolar bone. Controlling local infection and inflammation is crucial for successful outcomes, given the susceptibility of the periodontium to oral microflora. Current grafting methods often focus on bone regeneration but fall short in restoring periodontal tissues, highlighting the need for advancements in tissue engineering. The field has witnessed significant growth driven by advancements in fabrication techniques and material development, leading to the proposal of scaffold designs capable of guiding site-specific regeneration. However, clinical evidence supporting the efficacy of 3D-printed scaffolds remains limited, and regulatory challenges need to be addressed for the translation of tissue engineering therapies into clinical practice.
In recent developments, advanced imaging modalities integrated with biomaterial scaffolds show promise in optimizing scaffold composition and structure for effective periodontal regeneration. These technologies enable non-invasive tracking of in vivo scaffold degradation, though infrastructure improvements and stringent quality control measures are necessary, potentially increasing therapy costs. Despite our limited understanding of periodontal biology, emerging technologies like CRISPR hold potential in overcoming challenges associated with endogenous cell sources and severe inflammation. Collaborative efforts among biomaterial scientists, biologists, chemical engineers, and clinicians drive progress towards achieving functional regeneration of periodontal tissues, despite the inherent complexities associated with periodontitis and tissue regeneration.
Source of Funding
None.
Conflict of Interest
None.
References
- R Langer, JP Vacanti. Tissue engineering. Science 1993. [Google Scholar]
- A Kumar, SM Un-Nisar, A Zia. Tissue Engineering-The promise of regenerative dentistry. Biol Med 2011. [Google Scholar]
- M F Pittenger, A M Mackay, S C Beck, R K Jaiswal, R Douglas, J D Mosca. Multilineage potential of adult human mesenchymal stem cells. Science 1999. [Google Scholar]
- C M Misch. Autogenous bone: is it still the gold standard?. Implant Dent 2010. [Google Scholar]
- B L Eppley, W S Pietrzak, M W Blanton. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. J Craniofac Surg 2005. [Google Scholar]
- E Jimi, S Hirata, K Osawa. The current and future therapies of bone regeneration to repair bone defects. Int J Dent 2012. [Google Scholar]
- T Nakahara, T Nakamura, E Kobayashi, K Kuremoto, T Matsuno, Y Tabata. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament derived cells. Tissue Eng 2004. [Google Scholar]
- R T Kao, S Murakami, O R Beirne. The use of biologic mediators and tissue engineering in dentistry. Periodontol 2000. [Google Scholar]
- B M Seo, M Miura, S Gronthos, P M Bartold, S Batouli, J Brahim. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004. [Google Scholar]
- K Yamamiya, K Okuda, T Kawase, K Hata, L F Wolff, Yoshie H. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J Periodontol 2008. [Google Scholar]
- M Mohammadi, M A Shokrgozar, R Mofid. Culture of human gingival fibroblasts on a biodegradable scaffold and evaluation of its effect on attached gingiva: A randomized, controlled pilot study. J Periodontol 2007. [Google Scholar]
- H Kawaguchi, A Hirachi, N Hasegawa, T Iwata, H Hamaguchi, H Shiba. Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 2004. [Google Scholar]
- J C Ashworth. Optimising collagen scaffold architecture for enhanced periodontal ligament fibroblast migration. J Mater Sci Mater Med 2018. [Google Scholar]
- A Kato. Combination of root surface modification with BMP-2 and collagen hydrogel scaffold implantation for periodontal healing in beagle dogs. Open Dent J 2015. [Google Scholar]
- S Nakamura. Acceleration of bone regeneration of horizontal bone defect in rats using collagen-binding basic fibroblast growth factor combined with collagen scaffolds. J Periodontol 2019. [Google Scholar]
- S Zang. Injectable chitosan/β-glycerophosphate hydrogels with sustained release of BMP-7 and ornidazole in periodontal wound healing of class III furcation defects. Mater Sci Eng C 2019. [Google Scholar]
- H Abukawa, M Papadaki, M Abulikemu, J Leaf, JP Vacanti, LB Kaban. The engineering of craniofacial tissues in the laboratory: A review of biomaterials for scaffolds and implant coatings. Dent Clin North Am 2006. [Google Scholar]
- T Nakahara. A review of new developments in tissue engineering therapy for periodontitis. Tissue Eng Dent Clin North Am 2006. [Google Scholar]
- J Liu, J Ruan, MD Weir, K Ren, A Schneider, P Wang. Periodontal Bone-Ligament-Cementum Regeneration via Scaffolds and Stem Cells. Cells 2019. [Google Scholar]
- S Ivanovski, C Vaquette, S Gronthos, DW Hutmacher, PM Bartold. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res 2014. [Google Scholar]
- M Cho, WL Lin, RJ Genco. Platelet Derived growth factor modulated guided tissue regenerative therapy. J Periodontol 1995. [Google Scholar]
- VP Terranova, C Odziemiec, KS Tweden, DP Spadone. Repopulation of dentin surfaces by periodontal ligament cells and endothelial cells. Effect of basic fibroblast growth factor. J Periodontol 1989. [Google Scholar]
- JM Wozney, V Rosen. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 1998. [Google Scholar]
- TJ Sigurdsson, MB Lee, K Kubota. Periodontal repair in dogs: Recombinant bone morphogenetic protein 2 significantly enhances periodontal regeneration. J Periodontol 1995. [Google Scholar]
- NT Bennett, GS Schultz. Growth factors and wound healing: Biochemical properties of growth factors and their receptors. Am J Surg 1993. [Google Scholar]
- N Matsuda, RJ Genco, NM Kumar, MI Cho. Mitogenic, chemotactic and synthetic response of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol 1992. [Google Scholar]
- D Kaigler, JA Cirelli, WV Giannobile. The potential role of growth and differentiation factors in periodontal regeneration. J Periodontol 1996. [Google Scholar]
- O’brien. Biomaterials & scaffolds for tissue engineering. Mater Today 2011. [Google Scholar]
- QL Loh, C Choong. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng B Rev 2013. [Google Scholar]
- V Karageorgiou, D Kaplan. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005. [Google Scholar]
- H Jin, Y Zhuo, Y Sun. Microstructure design and degradation performance in vitro of three-dimensional printed bioscaffold for bone tissue engineering. Adv Mech Eng 2019. [Google Scholar]
- S Ivanovski, C Vaquette, S Gronthos, DW Hutmacher, PM Bartold. Multiphasic scaffolds for periodontal tissue engineering. J Dent Res 2014. [Google Scholar]
- M Yang, X Gao, Z Shen. Gelatin-assisted conglutination of aligned polycaprolactone nanofilms into a multilayered fibre-guiding scaffold for periodontal ligament regeneration. RSC Adv 2019. [Google Scholar]
- T Momose. Collagen hydrogel scaffold and fibroblast growth factor-2 accelerate periodontal healing of class II furcation defects in dog. Open Dent J 2016. [Google Scholar]
- CH Lee. Three-dimensional printed multiphase scaffolds for regeneration of periodontium complex. Tissue Eng 2014. [Google Scholar]
- CH Park. Prototype development for the periodontal model system with the spatial compartmentalization by the additive manufacturing. Appl Sci 2019. [Google Scholar]
- L Zhang, G Yang, N Blake, N Johnson, X Jia. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater 2019. [Google Scholar]
- E Bell. Strategy for the selection of scaffolds for tissue engineering. Tissue Eng 1995. [Google Scholar]
- N Thattaruparambil Raveendran, C Vaquette, C Meinert. Optimization of 3D bioprinting of periodontal ligament cells. Dent Mater 2019. [Google Scholar]
- Y Ma, Y Ji, G Huang. Bioprinting 3D cell-laden hydrogel microarray for screening human periodontal ligament stem cell response to extracellular matrix. Biofabrication 2015. [Google Scholar]
- S Sprio, E Campodoni, M Sandri. A graded multifunctional hybrid scaffold with superparamagnetic ability for periodontal regeneration. Int J Mol Sci 2018. [Google Scholar]
- CH Park, HF Rios, Q Jin. Biomimetic hybrid scaffolds for engineering human tooth-ligament interfaces. Biomaterials 2010. [Google Scholar]
- Introduction
- Background of Periodontal Regeneration Therapy
- Periodontal ligament‑derived cells
- Periosteal cells
- Gingival epithelium and fibroblast
- Bone marrow‑derived mesenchymal stem cells
- Scaffold biomaterials for periodontal regeneration
- Natural polymers
- Chitosan
- Hydrogel
- 3D-printed scaffolds
- Signalling molecules
- Bone Morphogenetic Proteins
- Insulin Like Growth Factor
- Transforming Growth Factor-β
- Periodontal Ligament Derived Growth Factor
- Scaffold Architectures and Fabrication Techniques
- Discussion
- Conclusion and Future Perspective
- Source of Funding
- Conflict of Interest
