- Visibility 140 Views
- Downloads 22 Downloads
- DOI 10.18231/j.johs.2024.036
-
CrossMark
- Citation
Improving life quality via mandibular reconstruction: Innovations and techniques – A literature review
Introduction
In order to maximize both functional and cosmetic results, mandibular abnormalities resulting from ablative surgery for malignant tumors of the head and neck region impact both form and function. This calls for a multidisciplinary approach. Complete reconstruction techniques necessitate the reconstruction of the width, height, and projection of the face. Reconstructive surgeons need to be skilled in replacing skeletal buttresses, restoring the external and internal soft tissue envelope, closing fistulas, and creating a foundation for dental rehabilitation in order to produce the best functional and cosmetic outcomes.[1] In addition to being crucial for speaking, deglutition, and mastication, the mandible also helps to stabilize the airway and plays an important part in determining the shape of the lower face. Therefore, in mandible repair, functional and cosmetic criteria are equally significant. Particular functional objectives include the maintenance of occlusion and preservation of tandem temporomandibular joint motion with maximum opening ability. Aesthetic targets include correcting submandibular soft tissue neck abnormalities, preserving anterior chin projection and lower facial height.Cancer is the main root cause of segmental mandible abnormalities. At the moment, vascularized bone grafts inserted using microvascular method represent the gold standard for mandibular repair. reconstruction of a mandibular segmental deficit using vascularized.In addition to providing well-vascularized tissue resistant to adjuvant radiation therapy and enabling osseointegrated implant dentistry, bone facilitates primary healing.[2] These days, imaging technology allow for the creation of 3-D models that can be used to practice an operation, assess the need for soft or hard tissue restoration and/or augmentation (if needed), or even act as a template for the custom fabrication of facial implants.[3]
Indication
If the patient's health allows, segmental mandibular deficiencies should be rebuilt.
Dental rehabilitation will be possible if the buccal sulcus is deep enough. If additional external skin replacement or a significant quantity of intraoral soft tissue restoration is needed, a second flap—either pedicled or free—may be necessary.[1]
Reconstruction using free or vascularized bone is necessary when a significant portion of the mandible is destroyed or absent. Bony segments on either side of the deformity can be stabilized with a reconstructive titanium plate.[4]
When healthy tissue with a strong blood supply surrounds a comminuted, otherwise healthy bone, it can be repaired using free, non-vascularized bone grafting.[5]
Contraindication
Anytime there is a developing infection in the area of the wound, reconstruction should be postponed as it might harm the graft.[6]
If the patient will soon be receiving radiation therapy as part of their cancer treatment, non-vascularized graft repair should not be done since the consequent reduction in the vascularity of the surrounding tissue significantly raises the risk of graft necrosis.[7]
A patient should not have fibula harvest if he does not have appropriate collateral circulation or "three-vessel runoff" to the foot.[8]
Classification of mandibular defects
Pavlov classification (1974) The first attempt at classifying mandibular defects was made by Pavlov (Russia) in the pre-free tissue transfer era.
Divided mandibular defects into 3 classes based on whether the remaining arch was left in 1, 2 or 3 fragments. (So, class 1 always involved a condyle and class 3 had 2 separate defects).
Subdivided into groups based on encroachment onto the mentum.
Further, split into subgroups based on the size of the defect.
Recognized the reconstructive and functional problem posed by absence of two key element- condyle (reconstructing a condyle is difficult and leads to functional impairment also) and mentum (degloving of chin or resection of entire submental musculature or mentum leads to ptosis of lower lip and chin– ‘Witch’s chin’ / ‘Andy Gump deformity’).
David et al. classification (1988)
Similar in spirit to HCL classification which was published shortly afterward in 1989 (described below).
Took into account many reconstructive difficulties mainly related to the resection of the condyle and the central segment.
Disadvantage- some bony defects (like short H, HCH, HCL, and HC of HCL classification) remained unclassified, soft tissue defects were not classified.
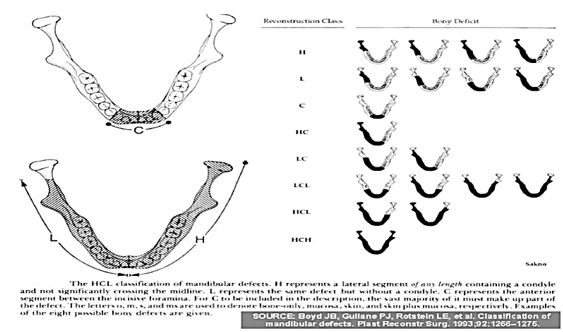
HCL classification by Jewer and Boyd et al. (1989)- modified by Boyd et al.(1993)
When first published in 1989, it used 3 UPPER case alphabets (H, C, L) to classify bony mandibular defects only, whereo ‘H’ – (Hemi-mandibulectomy) stands for lateral segment defect ‘of any length’ including the condyle, o ‘C’ – stands for Central defects including the ‘entire’ anterior segment (including 2 canines and 4 incisors) and, o ‘L’ – stands for Lateral defects excluding the condyle.
Boyd’s modified HCL classification included soft tissue defects by adding 3 lower case alphabets (o, m, s) as subscripts to the 3 uppercase alphabets (H, C, L) while classifying: o ‘o’ – no mucosal or skin component, o ‘m’ – mucosal component, o ‘s’ – skin component.
Urken et al CRBS classification (1991)
Its a comprehensive classification of composite oromandibular defects which includes ‘neurological deficits’ (8 possibilities) in addition to bony (20 possibilities) and soft tissue defects (22 possibilities).
Bone defects- Condyle, Ramus, Body, Symphysis (S-total, S H: superscriptH indicateshemisymphysis defect), superscript M to C/R/B/C- indicates marginal resection in that specific part of the mandible.
Soft tissue defectso Mucosa: L- labial, B– Buccal, SP– Soft Palate, FOM– Floor of Mouth; o T- Tongue, o C- Cutaneous. The above is again subdivided with various superscripts based on the location of the defect (for eg. anterior, posterior, lateral) in each structure.
Neurologic defects- N followed by subscript– IA– Inferior Alveolar, L– Lingual, H– Hypoglossal or F– Facial (with a superscriptB for Bilateral involvement).
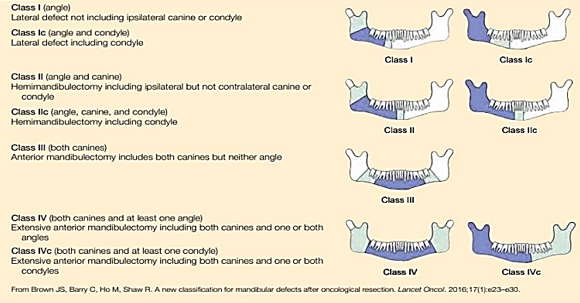
Brown classification
Timing of Reconstruction
In the past, proponents of a staged or delayed approach recommended a time of observation in order to ensure histologically clear bone margins before reconstruction or to keep an eye out for any signs of recurrent disease. But these days, it's commonly acknowledged because there is no chance that an early detection of recurrent illness will prevent an instant reconstruction from being carried out9. Before the development of microsurgical methods, it was essential to postpone reconstruction to give the wound bed time to heal and prepare for nonvascular bone grafting.[9]
For mandibular reconstruction, immediate reconstruction has a number of additional benefits over delayed reconstruction. Studies on health-related quality of life (QOL) have shown that prompt repair greatly enhances QOL and that the majority of patients favor instant reconstruction. Additionally, Boyd noted that patients undergoing vascularized bone flap repair lost an average of 4 days of life due to follow-up surgeries, while patients undergoing plate and soft tissue flap reconstruction lost 35 days.[10], [11], [12], [13], [14], [15]
Reconstructive Options Used for Reconstruction of Mandible
Modern mandibular reconstruction requires the surgeons to have many options at their expertise. Many reconstruction modalities have been attempted and reported. These modalities include reconstruction bars with or without pedicled myocutaneous flaps, alloplasts, free grafts including particulate or cortical bone, pedicled osteomyocutaneous flaps, and a variety of free vascularized bone flaps. No one method of reconstruction deals with all the variables affecting each patient with a mandibular defect. It is important to mention that one option is no reconstruction of the mandible.[16], [17]
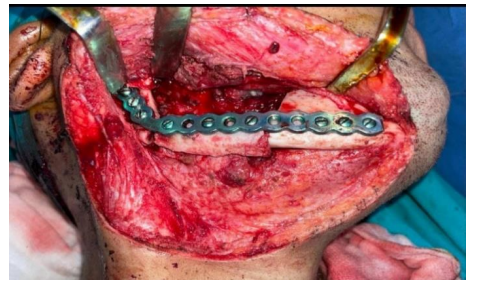
Reconstruction plates
Some form of rigid fixation is necessary for mandibular reconstruction to secure the remaining bone fragments and any grafts, which are used to provide adequate healing and maintain mandible position. Whichever plate is decided upon, it should be contoured before the resection to bridge the defect with an adequate amount of fixation on either side of it. Usually, two-point fixation on each side of the defect is minimal. MMF is necessary before the resection to ensure the occlusal relationship. Mandibular reconstruction plates and screws are the most widely used alloplastic devices for mandibular reconstruction. The most common metals used in the fabrication of these plates are stainless steel, vitallium, and titanium The perforated hollow titanium screw used in the titanium hollow osseointegrated reconstruction plate (THORP) reconstruction plate system permits bone ingrowth and osseointegration, which should strengthen the stability of the bone-screw contact.[18]
Instead of being compressed to the underlying mandible, the plate can be anchored to the interosseous screw by means of an expansion bolt located within the screw head. This reduces the chance of plate failure at the screw-bone interface by preventing pressure necrosis of the underlying bone.[19]
Methods for Replacing Autogenous Bone 1
Non-vascularized bone grafts
Vascularized free flaps Of the two, the following variables decide which technique to use:
The soft tissue environment's quality (the soft tissue bed is heavily scarred due to radiation exposure, prior graft failure, and infection).
The soft tissue's sufficiency (related lining and cover requirement)
Defect's dimensions and shape.
The surgeon's experience.
Non-vascularized bone grafts
This is used to treat minor mandibular abnormalities when there is little to no soft tissue loss. The bed where the bone graft is implanted has good blood flow. To ensure sufficient bone-to-bone contact, the periosteum surrounding the neighboring bone fragments is removed. The typical non-vascularized bone donor locations iliac crest and ribs are used as grafts. The rib can be divided or used as a full rib graft. Because there is no exposed cancellous bone, the rib transplant revascularizes relatively slowly, which makes the entire procedure less successful. Another preferred location for non-vascularized bone transplants is the iliac crest because it is easily accessible and has a rich supply of cancellous and cortical bone. It can be utilized to reconstruct the posterior and whole ramus in addition to the medium-sized abnormalities a segment of the mandible.[19]
Bone Graft Substitutes
Mowlem showed in 1944 the enhanced osteogenic potential of PBCM (cancellous bone grafts).[20] The capacity to produce anatomic mandibular reconstructions with sufficient height, symmetrical arch form and width, and enough support for dental implants are the benefits of using PBCM grafts. The drawbacks include of wound healing and remodeling resorption. Dehiscence and infection could still happen, which would mean the graft would be lost.[21] Wound dehiscence, tray exposure, and postoperative infection—all of which can result in the partial or complete loss of the graft—are complications associated with the use of metal or Dacron trays. The tray may need to be removed if it interferes with later prosthesis or implant rehabilitation.[22] Additional implantable possibilities include Delta [poly (L) lactide, 10% glycolide, and 5% poly (D) lactide], customized implants (75% methyl methacrylate styrene copolymer, 15% poly methyl methacrylate, and 10% barium sulfate), and MedPor (high density-permeable polymer).[23] Tideman et al.[24], [25] described a titanium mesh system for mandible reconstruction. A custommade titanium tray was designed to match the segment of mandible to be resected and fixed to the residual segments. Warnke et al.[26] created an individually made titanium-mesh cage filled with xenogenic bone mineral, recombinant human bone morphogenetic protein (BMP) and fresh bone marrow.
One of the most important developments in the creation of synthetic bone grafts has been the identification of bone morphogenic protein (BMP), the primary activator of bone induction. BMPs belong to the superfamily of transforming growth factor β.[27]
Vascularized Free Flaps
Healing in vascularized bone is possible even in situations where the recipient bed is damaged. On the other hand, nonvascularized bone heals by creeping substitution, or the resorption of old bone and deposition of new bone. microvascular-free Flaps enable osseointegration in a single main stage as well as long-term stability and dependability. Furthermore, no other reconstruction technique can match the vascularized bone flaps' capacity to create the sturdy arch required to restore form and function in cases of anterior mandibular abnormalities. A range of donor locations for soft tissue and vascular bone flaps have developed over the last ten years. In an ideal world, the bone would be long enough to span the deficiency and broad enough and tall enough to support endosteal implants and resist mastication. Furthermore, the restoration of function depends on soft-tissue repair. In order to restore function, the flap must therefore supply enough soft tissue if the lesion involves soft tissue.[28], [29], [30] Options include free fibula flap, radial forearm free flap, scapula free flap, anteriolateral thigh flap. The Pectoralis Major Myocutaneous Flap, Iliac Crest Free Flap, The Metatarsus Osteocutaneous Flap, Clavipectoral Osteomyocutaneous Free Flap.
Radiation therapy and osteoradionecrosis
Tissue responses can result from radiation therapy with both early and late onset.[31], [32] Typical radiation-induced fibrosis and bone demineralization are the late effects, along with a weakened immune system. The exposed osseous Due to decreased circulation, the structure is more susceptible to infection. In addition, radiation induces endarteritis, which leads to tissue hypoxia, hypocellularity, and hypovascularity. These factors cause tissue collapse, which leaves chronic wounds that do not heal.[33] ORN is defined as bone exposure within a radiation field that, despite conservative treatment, has not healed after three months.[34] Hirsch et al. examined the results and side effects of individuals receiving similarly repaired ORN and vascularized osseous flap reconstruction. individuals who underwent radiation treatment but did not experience ORN, in addition to unirradiated controls. Overall flap survival in their study was 88%, and there was no statistical difference in flap survival rates between ORN (86%), no ORN (87%), and controls (90%); there was also no difference in complication rates among the three groups. These findings imply that iliac crest, scapula, and fibula free flap transfers are reasonable choices for advanced mandibular ORN.[35]
Recent advances
Three-dimensional models can be produced thanks to technological advancements in rapid prototyping and medical imaging. When osteoradionecrosis has previously necessitated the removal of the mandible or destroyed it, a With a digitally generated "virtual" mandibular arch that is based on mirroring or a CT dataset that has a suitable occlusal connection to the maxilla, a reconstructive surgeon can design a plate either before or during surgery that will give the patient the best possible post-operative occlusion. In order to optimize bone-to-bone contact and encourage a robust bony union, the surgeon can save time by using templates for contouring osteotomies created from three-dimensional modeling of the bone transplant.[36]
Transport Disc Distraction Osteogenesis (TDDO) A method called transport disc distraction osteogenesis (TDDO) is applied for mandibular reconstruction. Next to the defect, a section of bone is sliced, and moved by a mechanical device across the fault gradually. Between the two bone segments, new bone grows. The term "transport disc" refers to the segment of bone that is being transferred or transported.[37]
TDDO has also been reported to provide sufficient bone to allow dental implant placement, an important functional outcome.[38] Only restoration of relatively straight-line defects—such as those involving the mandibular body—is susceptible to transport distraction. This is due to the fact that the regenerating connective tissue stroma determines the form of the rebuilt tissue. Thus, if it is necessary to reconstruct a defect of the symphysis, the best plan is to create transport discs from the right and left posterior stumps of the mandible and move them toward the symphysis.
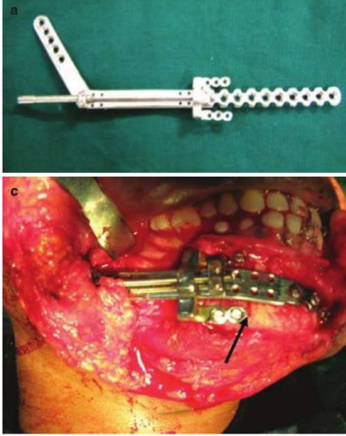
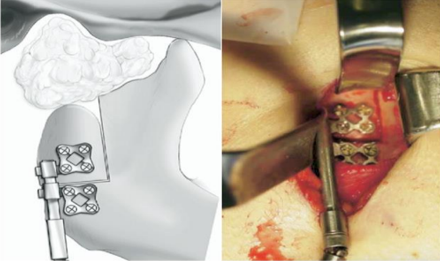
Modular Endoprosthesis
Modular endoprosthetic reconstruction has gained popularity as a standard procedure in limb-sparing surgery throughout the last ten years.[39] This method places a strong emphasis on removing any diseased bone and replacing it with an artificial device cemented into the remaining bone. A modular system allows for flexibility in the rebuilding of different fault sizes by combining standardised units. With reported 15-year survival rates of 94%, endoprostheses fixed with bone cement have demonstrated long-term stability and satisfactory function in long bones.[40] After resection, an endoprosthesis is a metallic device that is attached into the mandibular stump's medullary cavity. Screw fixing is not required. Modules can be used to bridge the bone gap's changeable length that enable precise reconstructions in three dimensions. The locking mechanism connects the modules. In the initial designs, bone cements were used to cement stems into the medullary area.
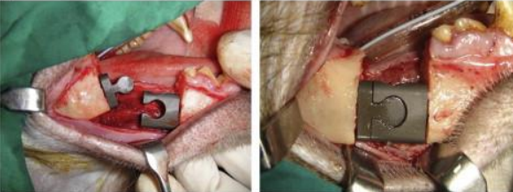
Published animal research describe the outcomes of replacing the ramus/condyle and mandibular body.
Tissue-Engineering
The goal of the multidisciplinary area of tissue engineering is to design biological replacements and therapeutic approaches that preserve, replace, or enhance biological functions. It does this by fusing the principles of biology, engineering, and material sciences. Growth elements as well as adequate regeneration can be induced by smad proteins, such as bone morphogenic proteins, and platelet rich plasma osteoprogenitor cells formed on a recipient bed. It is possible to repair functional tissue using autologous patient cells thanks to the tissue engineering approach. Therapeutic alternatives for benign conditions include free bone transplants and tissue engineering methods.
Tissue engineering can offer solutions
The shape can be controlled by customised scaffolds on the basis of CAD/CAM and radiographic imaging data.
Bone can be grown in muscular environments and then be harvested with reduced morbidity. An ideal site for such a bone flap prefabrication is the latissimus dorsi muscle.[41]
In theory, tissue engineering a graft at the defect location would be preferable to previous approaches; however, this would necessitate immobilizing the mandible during the healing phase. Other than the technical issues Unknown effects on different tissue cells and even straightforward carcinogenic effects are a concern. This is one of the primary causes of the current lack of widely accessible morphogenetic growth factors. For epithelial cells, at least, autogenous growth factors, such as those found in platelets, are primarily mitogenetic and are not known to be oncogenetic.[42]
Dental rehabilitation
Dental implants are crucial to the restoration of masticatory function because they maintain the natural bone by imitating physiologic bone repair and enable the attachment of prosthesis.[43]
In autogenously transplanted bone, there were high rates of soft-tissue inflammation near implants, and it was discovered that both the transplantation technique and the donor site selection appeared to have an impact on the development of inflammation around implants.[44] Over a mean follow-up period of three years, Foster et al.[45] reported an implant success rate of 99% for vascularized bone flaps against 82% for non-vascularized bone grafts. There isn't a discernible difference between implant loss rates in exposed and Free bone flaps without radiation.[46], [47], [48] Primary implants can be inserted during reconstructive and ablative surgery, while secondary implants can be inserted subsequently.[49]
Conclusion
For patients with a variety of mandibular abnormalities, mandibular reconstruction is a vital and developing area of maxillofacial surgery that is crucial to the restoration of both form and function. We have investigated a number of cutting-edge methods through this thorough literature study, such as free tissue transfers, vascularized bone grafts, and computer-aided design and manufacture (CAD/CAM) technologies. For various sorts of defects—whether from trauma, tumor resection, or congenital anomalies—each approach provides unique benefits. The selection of reconstruction technique is contingent upon various criteria, including defect location, size, and patient-specific considerations. This underscores the significance of an individualized approach in attaining the best possible results. Further improvements in patient rehabilitation and quality of life are anticipated as surgical techniques and technology continue to evolve, underscoring the critical role that continuing research and multidisciplinary collaboration play in advancing field of mandibular reconstruction.
Source of Funding
None.
Conflict of Interest
None.
References
- BP Kumar, V Venkatesh, KA Jeevan Kumar. Mandibular Reconstruction: Overview. J Maxillofac Oral Surg 2016. [Google Scholar]
- PC Neligan. Head and Neck Reconstruction. Plastic Reconst Surg 2013. [Google Scholar]
- S Stosić. Mandibular reconstruction - State of the art and perspectives. Vojnosanitetski Pregled 2008. [Google Scholar]
- BB Pickrell, AT Serebrakian, RS Maricevich. Mandible Fractures. Semin Plast Surg 2017. [Google Scholar]
- ZH Ren, TF Fan, S Zhang, HJ Wu. Nonvascularized Iliac Bone Reconstruction for the Mandible Without Maxillofacial Skin Scarring. J Oral Maxillofac Surg 2020. [Google Scholar]
- GK Parise, MI Guebur, G Ramos, AK Groth, A Da Silva, LM Sassi. Evaluation of complications and flap losses in mandibular reconstruction with microvascularized fibula flap. Oral Maxillofac Surg 2018. [Google Scholar]
- A Vincent, R Sawhney, Y Ducic. Perioperative Care of Free Flap Patients. Semin Plast Surg 2019. [Google Scholar]
- L Oxford, Y Ducic. Use of fibula-free tissue transfer with preoperative 2-vessel runoff to the lower extremity. Arch Facial Plast Surg 2005. [Google Scholar]
- W Lawson, LJ Loscalzo, SM Baek. Experience with immediate and delayed mandibular reconstruction. Laryngoscope 1982. [Google Scholar]
- PG Cordeiro, DA Hidalgo. Conceptual considerations in mandibular reconstruction. Clin Plast Surg 1995. [Google Scholar]
- A Baker, J Mcmahon, S Parmar. Immediate reconstruction of continuity defects of the mandible after tumor surgery. J Oral Maxillofac Surg 2001. [Google Scholar]
- X Li, K Zhu, F Liu, H Li. Assessment of quality of life in giant ameloblastoma adolescent patients who have had mandible defects reconstructed with a free fibula flap. World J Surg Oncol 2014. [Google Scholar]
- DT Netscher, RA Meade, CM Goodman. Quality of life and disease specific functional status following microvascular reconstruction for advanced (T3 and T4) oropharyngeal cancers. Plast Reconstr Surg 2000. [Google Scholar]
- EA Weymuller, B Yueh, F Deleyiannis. Quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2000. [Google Scholar]
- JB Boyd, RS Mullholland, J Davidson. The free flap and plate in oromandibular reconstruction: long-term review and indications. Plast Reconstr Surg 1995. [Google Scholar]
- PJ Gullane, H Holmes. Mandibular reconstruction new concept. Arch Otolaryngol Head Neck Surg 1976. [Google Scholar]
- ML Urken. Composite free flaps in oro-mandibular reconstruction. Arch Otolaryngol Head Neck Surg 1991. [Google Scholar]
- T Vuillemin, J Raveh, F Sutter. Mandibular reconstruction with the titanium hollow screw reconstruction plate (THORP) system: evaluation of 62 cases. Plast Reconstr Surg 1998. [Google Scholar]
- MA Schusterman, KP Reece, SS Kroll. Use of the A-O plate for mandibular reconstruction in cancer patients. Plast Reconstr Surg 1991. [Google Scholar]
- MD Dmd, E Keller. Iliac Crest Grafting for Mandibular Reconstruction Deepak Kademani. Mayo Clinic College Med 2006. [Google Scholar]
- TW Albert, JD Smith, E Everts, TA Cook. Dacron mesh tray and cancellous bone in reconstruction of mandibular defects. Arch Otolaryngol Head Neck Surg 1980. [Google Scholar]
- LK Cheung, N Samman, TW Chow, R Clark, H Tideman. A bone graft condensing syringe system for maxillofacial reconstructive surgery. Br J Oral Maxillofac Surg 1997. [Google Scholar]
- Y Kinoshita, M Kobayashi, T Hidaka, Y Ikada. Reconstruction of mandibular continuity defects in dogs using poly (Llactide) mesh and autogenic particulate cancellous bone and marrow: preliminary report. J Oral Maxillofac Surg 1997. [Google Scholar]
- N Samman, LK Cheung, H Tideman. Functional reconstruction of the jaws: new concepts. Ann R Australas Coll Dent Surg 1996. [Google Scholar]
- H Tideman, N Samman, L K Cheung. Functional reconstruction of the mandible: a modified titanium mesh system. Int J Oral Maxillofac Surg 1998. [Google Scholar]
- PH Warnke, IN Springer, J Wiltfang, Y Acil, H Eufinger, M Wehmo¨ller. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004. [Google Scholar]
- AJ Celeste, JA Iannazzi, RC Taylor, RM Hewick, V Rosen, EA Wang. Identification of transforming growth factor beta family members presentin boneinductive protein purified from bovine bone. Proc Natl Acad Sci 1990. [Google Scholar]
- DA Hidalgo. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg 1989. [Google Scholar]
- DA Hidalgo. Aesthetic improvements in free-flap mandible reconstruction. Plast Reconstr Surg 1991. [Google Scholar]
- DA Hidalgo, A Rekow. Review of 60 consecutive fibula free flap mandible reconstructions. Plast Reconstr Surg 1995. [Google Scholar]
- MA Pogrel, S Podlesh, JP Anthony. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg 1997. [Google Scholar]
- G Granstro, M Jacobsson, A Tjellstro. Titanium implants in irradiated tissue: benefits from hyperbaric oxygen. Int J Oral Maxillofac Imp 1992. [Google Scholar]
- RE Marx. A new concept in the treatment of osteoradionecrosis. J Oral Maxillofac Surg 1983. [Google Scholar]
- DS Alealam, M Nuara, J Christian. Analysis of outcomes of vascularized flap reconstruction in patients with advanced mandibular osteoradionecrosis. Otolaryngol Head Neck Surg 2009. [Google Scholar]
- BT Goh, S Lee, H Tideman, PJ Stoelinga. Replacement of the condyle and ascending ramus by a modular endoprosthesis in Macaca fascicularis part 1: a clinical and radiographic study. J Oral Maxillofac Surg 2009. [Google Scholar]
- BD Foley. Mandibular reconstruction using computer-aided design and computer-aided manufacturing: an analysis of surgical results. J Oral Maxillofac Surg 2013. [Google Scholar]
- PD Costantino, G Shybut, CD Friedman, HL Pelzer, M Masini, ML Shindo. Segmental mandibular regeneration by distraction osteogenesis: an experimental study. Arch Otolaryngol Head Neck Surg 1990. [Google Scholar]
- R Gonza´lez-Garcı´a, L Naval-Gı´as. Transport osteogenesis in the maxillofacial skeleton: the outcomes of a versatile reconstruction method following tumor ablation. Arch Otolaryngol Head Neck Surg 2010. [Google Scholar]
- MM Malawer, LB Chou. Prosthetic survival and clinical results with use of large segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am 1995. [Google Scholar]
- U Riede, M Luem, T Ilchmann, M Eucker, PE Ochsner. The M.E Mu¨ller straight stem prosthesis: 15 year follow-up. Survivorship and clinical results. Arch Orthop Trauma Surg 2007. [Google Scholar]
- S Gronthos. Reconstruction of human mandible by tissue engineering. Lancet 2004. [Google Scholar]
- M Heliotis, KM Lavery, U Ripamonti, E Tsiridis, D Silvio. Transformation of a prefabricated hydroxyapatite/os1teogenic protein-1implant into a vascularised pedicled bone flap in the human chest. Int J Oral Maxillofac Surg 2006. [Google Scholar]
- PJ Boyne. Transplantation, implantation and grafts. Dent Clin North Am 1971. [Google Scholar]
- F Blake, M Bubenheim, M Heiland. Retrospective assessment of the periimplant mucosa of implants inserted in reanastomosed or free bone grafts from the fibula or iliac crest. Int J Oral Maxillofac Imp 2008. [Google Scholar]
- RD Foster, JP Anthony, A Sharma, MA Pogrel. Vascu1larized bone flaps versus nonvascularized bone grafts for mandibular reconstruction: an outcome analysis of primary bony union and endosseous implant success. Head Neck 1999. [Google Scholar]
- T Weischer, C Mohr. Ten-year experience in oral implant rehabilitation of cancer patients: treatment concept and proposed criteria for success. Int J Oral Maxillofac Imp 1999. [Google Scholar]
- R Werkmeister, D Szulczewski, P Walteros-Benz, U Joos. Rehabilitation with dental implants of oral cancer patients. J Cranio maxillofac Surg 1999. [Google Scholar]
- RJ Shaw, AF Sutton, JI Cawood, RA Howell, D Lowe, JS Brown. Oral rehabilitation after treatment for head and neck malignancy. Head Neck 2005. [Google Scholar]
- A Sclaroff, B Haughey, WD Gay, R Paniello. Immediate mandibular reconstruction and placement of dental implants at the time of ablative surgery. Oral Surg Oral Med Oral Pathol 1994. [Google Scholar]
How to Cite This Article
Vancouver
Agrawal AK, B AA, Ajmera S, Sharma AK, Sharma V, Meena P. Improving life quality via mandibular reconstruction: Innovations and techniques – A literature review [Internet]. J Orofac Health Sci. 2025 [cited 2025 Sep 09];11(4):178-185. Available from: https://doi.org/10.18231/j.johs.2024.036
APA
Agrawal, A. K., B, A. A., Ajmera, S., Sharma, A. K., Sharma, V., Meena, P. (2025). Improving life quality via mandibular reconstruction: Innovations and techniques – A literature review. J Orofac Health Sci, 11(4), 178-185. https://doi.org/10.18231/j.johs.2024.036
MLA
Agrawal, Ankush Kumar, B, Aravind Anto, Ajmera, Shruti, Sharma, Amit Kumar, Sharma, Vikram, Meena, Pooja. "Improving life quality via mandibular reconstruction: Innovations and techniques – A literature review." J Orofac Health Sci, vol. 11, no. 4, 2025, pp. 178-185. https://doi.org/10.18231/j.johs.2024.036
Chicago
Agrawal, A. K., B, A. A., Ajmera, S., Sharma, A. K., Sharma, V., Meena, P.. "Improving life quality via mandibular reconstruction: Innovations and techniques – A literature review." J Orofac Health Sci 11, no. 4 (2025): 178-185. https://doi.org/10.18231/j.johs.2024.036
